Fission: heavy nuclei split after a neutron capture
Most nuclei change little when they undergo radioactive decay, retaining all or almost all of their constituent protons and neutrons. In rare cases however, heavy and unstable nuclei can break in two: a process known as nuclear fission.
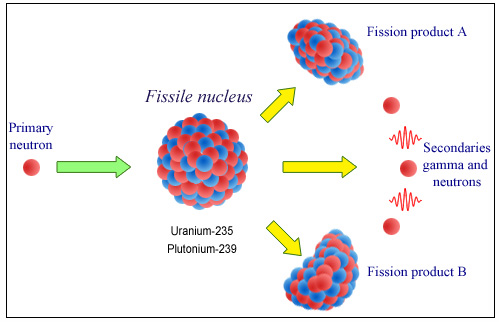
Nuclear fission
The absorption of a neutron by a fissile nucleus causes the nucleus to split into two radioactive fragments, referred to as the fission products, and two or three secondary neutrons.
© IN2P3
Spontaneous nuclear fission is an extremely rare phenomenon. Much more common is the fission induced by the capture of a neutron, a nuclear reaction which can be considered as a disintegration into two fragments of the nucleus, triggered by this capture. The neutron is the ideal projectile to use in this sort of reaction, as its absence of charge allows it to be easily absorbed by the heavy nucleus.
The heavy nuclei in which such a splitting occurs are known as ‘fissile‘, and only a handful of these unstable elements exist. The only fissile nucleus still present in nature is uranium 235, a rare isotope of uranium with a half-life of 700 million years.
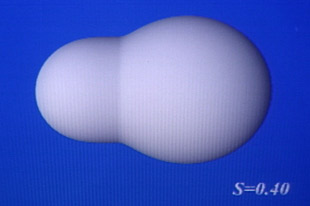
The splitting of a heavy nucleus
This computer-generated image shows the transformation of a single sphere into two smaller spheres without any volume being lost. The process shown above is similar to that of nuclear fission, where a nucleus deforms and splits into two nuclei of similar (though unequal) size. This image is an oversimplification, that does not show the neutrons and protons that make up the nucleus, and the emitted outgoing neutrons.
© CNRS- MEDARD Laurence
For almost all nuclei, the capture of a neutron is a mere capture. If the nucleus is fragile, however, owing to its size or composition, the arrival of an intruder may destabilize it to the point of breaking into two fragments.
Nuclear fissions occur also with the nuclei of plutonium 239, an isotope of plutonium. This important isotope is created from uranium nuclei in nuclear reactors, and has many of the properties of uranium 235.
Uranium 235 and plutonium 239 are fissionable by slow neutrons. The absorption of fast-moving neutrons is an effective way of setting off fission in an handful of other heavy nuclei. In this context, ‘fast-moving’ refers to speeds of the order of 10,000 kilometres per second: a speed sufficient to induce fission in nuclei of uranium 238, the most abundant isotope of uranium. Most other fissile elements are artificial; created in laboratories or specially-designed reactors with nuclei even heavier than those of uranium or plutonium.

Exploding with atomic energy …
Atomic energy, was the denomination given in the 1950’s for nuclear energy. It was synonym for vitality and trendiness as can be seen in this advertising. Even though this Zoé soda contains no radioactive substances, the posters claim that it bestows ‘atomic energy’ on those who drink it.
© ANDRA
Without the ability to trigger chain reactions, however, fission would at best have been an interesting footnote to the description of radioactivity. All the good, bad and so important applications of nuclear fission are a result of this vital self-sustaining quality, a quality that arises from the fact that the splitting of a nucleus is accompanied by the release of several free neutrons. These secondary neutrons have the ability to spark off other fission reactions which will, in turn, perpetuate the cycle by emitting other such neutrons. The total amount of energy released is no longer of the order of individual nuclei, as substantial amounts of matter can be used up in the process. These chain reactions can then either be used to create massive explosions (as in atomic bombs) or a controlled supply of energy (as can be seen in the reactors used in nuclear power plants).
On the positive side, nuclear fission has provided humanity with a virtually inexhaustible energy source. The inevitable formation of radioactive isotopes and therefore of radioactive waste is also a aspect of nuclear energy.
Learn more :
Articles on the subject « Nuclear Fission »
Chain Reaction
From one single fission to fissions on a massive scale Nuclear fission emits a lot of energy on t[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission fragment
Chronicle of the life of a fission fragment … The fission of a nucleus into two fragments i[...]
Fission products
The ashes of nuclear fission Fission products are the remains of a heavy uranium or plutonium nuc[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Short-lived Fission Products
Most fission products vanish rather rapidly The vast majority of the radioactive fission products[...]
Long-lived Fission Products
A handful of tough but little radioactive isotopes … The more a nucleus is long-lived, the [...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Plutonium Isotopes
Plutonium-239 but also 238, 240, 241, 242 … Plutonium isotopes are produced by neutron capt[...]
Minor Actinides
Neptunium, americium and curium Minor actinides constitute a very small minority of high activity[...]