Primordial and stellar nucleosynthesis
The Universe hasn’t always existed, It was born a little over 13 billion years ago. At the beginning of the Universe, atoms did not exist. « Nucleosynthesis » is the term used to call the processes of synthesis of chemical elements in the Universe. There are mainly two types of nucleosynthesis: primordial nucleosynthesis and stellar nucleosynthesis.

Orion Nebula
Orion Nebula M42, visible in winter with binoculars. It is a cloud of gas in space lit by the light of several stars. The red color is due to the presence of hydrogen and nitrogen, the blue color to the presence of oxygen.
© F.Boulay
Primordial Nucleosynthesis
The synthesis of chemical elements occurring at the very beginning of the Universe, a few minutes after the Big Bang 13.8 billion years ago, is called the « primordial nucleosynthesis ». Calculations and observations, in particular measurements of the abundances of chemical elements on old stars, more than 12 billion years old, show that the Universe at its birth was composed mainly of hydrogen atoms (whose nuclei contain 1 proton) and helium (whose nucleus contains 2 protons and two neutrons). There was approximately 75 wt% hydrogen and 25% helium. This agreement between theory and observation constitutes one of the proofs in favor of the Big Bang model. So there were no other chemical elements, no oxygen for example, just hydrogen, helium and some traces of very light chemical elements (lithium, beryllium). Today, research on primordial nucleosynthesis is focused on the enigma of lithium-7 abundance, see for example the work of the Nuclear Astrophysics Group of GANIL.
Today there are more than 250 different kinds of atoms on Earth whose nuclei contains less than 92 protons and less than 146 neutrons. A second process of synthesis of chemical elements necessarily followed primordial nucleosynthesis. This process called « stellar nucleosynthesis » is in fact constantly at work throughout the Universe and is particularly responsible for the emergence of life in our solar system (4.6 billion years old « only ») thanks to the synthesis of chemical elements such as oxygen, carbon or even nitrogen.
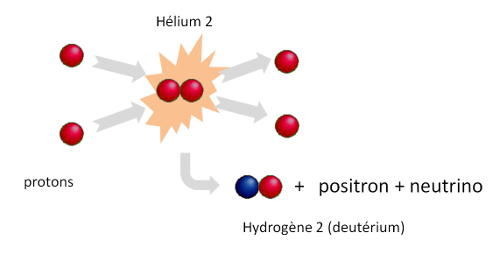
The pp1 reaction
The reaction called « pp1 » is the first fusion reaction. Two protons merge to form a deuterium nucleus which consists of a proton and a neutron.
© GANIL
Stellar Nucléosynthesis
After decades of research, researchers have understood that many chemical elements have been formed in the cores of stars and some in interstellar media (between stars). To come to this conclusion, they had to understand how chemical elements can transform into other chemical elements. This happens at the level of atoms nuclei. There is, of course, radioactivity, which makes it possible to transform a chemical element into another chemical element, such as potassium 40 into calcium 40 for example. Above all, there are the nuclear fusion reactions which combine two light nuclei of atoms to form a new, heavier nucleus. It is the intense heat in the cores of stars that allows these fusion reactions to occur. This is why one speaks of a thermonuclear fusion reaction. Our star, the sun, is the seat of such reactions. The most important fusion reaction in the sun is called the pp1 reaction.
La réaction pp1
The sun is made up primarily of hydrogen and helium. The nucleus of the hydrogen atom is made up of a single proton. In the sun core, due to the temperature of 15 million degrees and the thermal agitation that prevails there, collisions between two protons frequently occur which merge them to form transitory helium-2 nuclei ( composed of 2 protons). This helium 2 nucleus is not stable, and it dissociates almost instantaneously (in 10-21 seconds) into two protons, a waste of time … Hans Bethe, an American physicist born in Strasbourg and Nobel Prize in 1967, proposed the first in 1938 the nuclear reaction explaining the origin of the energy produced by the sun. He showed that some of this « virtual » helium 2 can make a « beta plus » radioactive decay and transform into a stable nucleus of deuterium (the isotope 2 of hydrogen, a nucleus of hydrogen. containing a proton and a neutron).
This fusion + radioactive decay reaction is called the pp1 reaction. Its probability is very low, but it is sufficient to explain the energy generated by the sun, the longevity of the sun, and the evolution of stars of comparable mass. These deuterium nuclei, which contains neutrons, will undergo later other fusion reactions and be the source of helium.
Other articles on the subject « Radioactive nuclei »
Map of Nuclei
Map of stable and unstable nuclei The progress made in our understanding of the subatomic world o[...]
Stability Valley
Beta decay : nuclei getting slimmer to achieve stability The nucleus mass is related to its inter[...]
Nuclear Forces
Three nuclear forces and their hierarchy Three types of force act alongside each other inside a n[...]
Mecanisms of Radioactivity
The way radioactivity works and its origins The impressive range of half-lives that exist, extend[...]
α decay : tunnel effect
Particle and wave: an effect of quantum mechanics The great age of uranium and thorium nuclei tha[...]
Alphas with gammas
Gamma rarely accompanies alpha decays It is surprising that one can hold with hands a sample of p[...]
β decay : weak forces
The forces which allow a nucleus to emit beta electrons Beta decay (β) and electronic capture cha[...]
Weak Forces
A special and fascinating fundamental interaction A third force is at work in the nucleus next to[...]
Nucleosynthesis (continued)
Mechanisms of atomic nuclei formation Most of the nuclei of atoms that make up our daily life wer[...]