The access key to the fuel of modern nuclear power plants … and to atomic weapons
The uranium fuel used in power boiling or pressurised water reactors should contain around 4% of the fissile uranium-235 isotope, a substantial increase from the concentration of 0.7% that exists in natural uranium ore. The weapon-grade uranium used to manufacture atomic bombs, has a much higher concentration, above 90%
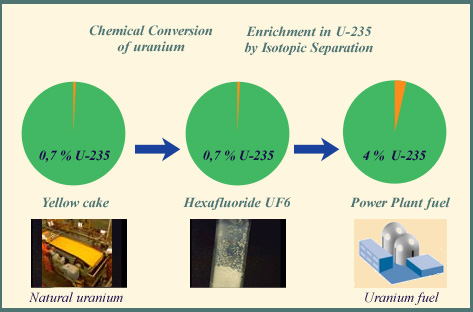
From natural uranium to power plants fuels
The first step of uranium enrichment by isotopic separation is its conversion to uranium hexafluoride. This chemical compound, shown above in the middle, becomes gaseous once it is heated. It is this gaseous uranium that is then enriched in fissile isotope 235, in isotopic separation installations in order to obtain the nuclear fuels of modern nuclear power plants.
© IN2P3
Uranium enrichment is a complex and extremely difficult procedure. As uranium 235 and 238 both have identical chemical properties, nuclear engineers have to rely on minor physical differences that exist in order to modify the isotope composition of uranium. Gaseous diffusion and ultracentrifugation, the two procedures which are used on an industrial scale, both exploit the 1.26% mass difference which separates the two isotopes.
In both of these procedures uranium must first be converted into uranium hexafluoride, a compound which becomes gaseous when heated to over 80 degrees Celsius. This chemical is highly corrosive, and therefore its manufacture and handling present hazards and require precautions.

Uranium conversion
A molecule of uranium hexafluoride contains six atoms of fluorine for every atom of uranium. In order to obtain this molecule, one first produces molecules of uranium tetrafluoride – containing four atoms of fluorine per uranium atom. In a second step the tetrafluoride is transformed into hexafluoride. The photograph shows a view of the Comurhex-Pierrelatte factory where the tetrafluoride to hexafluoride transition takes place. The manufacture and use of this highly corrosive substance require strict safety precautions as well as highly specialised equipment.
© AREVA / LESAGE (PHILIPPE)
The method of gaseous diffusion is the older of the two. It relies on the fact that uranium 235 can pass through a porous membrane slightly more quickly than its heavier rival isotope. Once the gas passes through a large number of diffusers, the uranium gas can be enriched to the required 3 to 5% level in uranium-235. The main disadvantage of this technique, however, is that it requires a very large amount of energy.
Ultracentrifugation, is a technique developed after the Second World War. It involves spinning a hollow cylinder containing uranium gas at very high speeds. The centrifugal force drives the heavier hexafluoride U-238 molecules more to the inner wall of the cylinder, beginning an isotopic separation. The hexafluoride gas must pass through thousands of centrifuges for its composition to reach the appropriate 3 to 5 % standard. The process of ultracentrifugation consumes little energy compared to gaseous diffusion.
A third procedure exists, but has not yet gone beyond the laboratory stage. A laser can be tuned to selectively ionise the molecules of uranium 235 at the core of a very hot metallic gas. These molecules can then be swept aside by an electric field. In principle this innovative process requires little energy to be performed and can be carried out in one step, but is handicapped by technical problems that are not solvable with current technology and materials.
Nuclear reactors in the United States are currently supplied with uranium obtained from dismantled atomic bombs. The United States Enrichment Corporation has a monopoly on the recycling of over 500 tons of highly enriched uranium fuel obtained from the arsenals of the ex-USSR. The weapon-grade uranium painfully enriched at a high cost is diluted with low grade natural uranium, like Ulysse’s Penelope destroying during the night the canvas woven during the day.
Every year, some 34 million SWU (separative work units) are carried out worldwide – enough to power 340 reactors producing 0.9 Gigawatts . Four large bodies dominate this field: AREVA, the URENCO consortium (involving the United Kingdom, the Netherlands and Germany), USEC in the United States and AEFA in Russia. Centrifuge procedures today account for up to two thirds of this enriched uranium.
. Four large bodies dominate this field: AREVA, the URENCO consortium (involving the United Kingdom, the Netherlands and Germany), USEC in the United States and AEFA in Russia. Centrifuge procedures today account for up to two thirds of this enriched uranium.
Enrichment generates, as a counterpart, substantial quantities of depleted uranium – uranium containing less than 0.2% of uranium 235 – every year. In 2004, for instance, over 8,000 tons of this uranium were produced in France. The radioactivity of this depleted uranium, similar to that of natural uranium, is low and not dangerous.
NEXT : Gaseous diffusion
NEXT : Ultracentrifugation
Other articles on the subject « Nuclear Fuel »
Nuclear Fuel Cycle
The front-end and the back-end of the cycle… The nuclear fuel cycle includes all nuclear op[...]
Cycle Front End
From the extraction of uranium to the fuel fabrication The front-end of the nuclear cycle is the [...]
Ultracentrifugation
A separation process economical in energy, but proliferating The principle of centrifuges has lon[...]
Gaseous Diffusion
The first enrichment process, a large consumer of electricity The gaseous diffusion process was t[...]
PWR fuel assemblies
Fuel assemblies in the core of PWR Reactors The core of a pressurized water reactor (PWR) is cont[...]
Reactor fuel layout
Fuel assemblies in the core of nuclear reactors The nuclear fuel assemblies, that vary from one r[...]
Plutonium Use
A fissile element produced in nuclear reactors In February 1941 the American physicist Glenn Seab[...]
Pu : Fuel, bomb or Waste ?
Plutonium dark and bright sides … Sensitive material, like the roman god Janus, plutonium h[...]
MOX fuels
Introducing plutonium in nuclear fuels For energy production, plutonum is gold ! One gram of plut[...]
Thorium Fuels
Uranium 233: a fissile nucleus made from thorium Thorium is more abundant than uranium in the Ear[...]