Radioactive atoms spying on the living …
The radioactive atoms, the radioisotopes are at the core of nuclear medicine. These unstable atoms behave like ordinary stable atoms apart from the unique and extremely short moment when they emit their radiation. But, their chemical and physical properties are those of the species to which they belong. They bind like them with other atoms to form molecules.
The alpha, beta, or gamma rays they emit during their fugitive radioactive decay signal their presence. Tiny objects of the infinitely small, radioisotopes are innumerable even in minute proportion in the matter. Thanks to the rays they emit, they can be located using good detectors. They are the spies of the living, the tools of diagnosis.
In therapy, the rays are used as weapons. One would shell tumors with an external source or by means of radioactive implants located near the malignant cells.
The use of radioisotopes for diagnosis is by far the most widespread.
Ever since the 1934 discovery of artificial radioactivity, doctors have been armed with a panoply of radioactive isotopes for use as markers or tracers. Thanks to these radioactive isotopes it is now possible to follow the path of a single atom or chemical element around the body without disturbing its physical, chemical or biological behaviour.
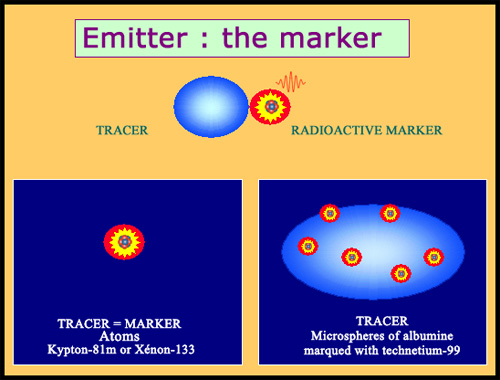
Markers and tracers
In biology and medicine, nuclear tracers are usually radiopharmaceutical products whose molecules contain a radioactive element – a marker. The emissions of radiations by such atoms allow to follow the path or the metabolism of these tracers in the body. Tracers can be reduced to a marker itself : this is the case of krypton 81m or xenon 133 , noble gases used for lung scans. Markers can be also attached to objects other than molecules, such as albumin microspheres.
© IN2P3
Such medical tests involve administering a radioactive tracer, chosen carefully for its ability to follow a metabolism or provide information about the working of a given organ. The tracer can be an individual atom (for instance iodine 123), a marked molecule (such as a diphosphonate marked with technetium 99m), an hormone or even an antibody marked with an isotope. The isotope has to be chemically attached to the relevant molecule without altering its properties and metabolism. The bond must also be a solid one in order to follow the molecule and not an eventually shaken off radioactive atom.
Radiopharmaceutical products (molecules containing radioactive isotopes) are generally inserted intravenously, though some can also be inhaled or even swallowed.
When it comes to internal body scans, the game is to localize the radiopharmaceutical product inside the body from an external detection while exposing the body to a minimal dose of radiation. Gamma emitters are therefore the ideal radioelements : gamma rays are relatively low ionisers and simultaneously penetrative enough to be detected outside of the body. Another important property of the radioelement is its half-life, which has to be long enough to follow the biological process in question and yet short enough to avoid any unnecessary exposure.
Technetium 99m is by far the most commonly used radioelement (used in 80-90% of all scintigraphy scans), as it allows for the exploration of numerous body parts and emits only gamma rays whose energy (of 140 keV) is well adapted to the gamma-camera detectors. Beta-plus (positrons) emitters are used in the field of ‘positron emission tomography’ (PET scans). In the field of metabolic therapy, beta-minus emitters are used to deliver a highly localized dose of radiation.