Proton therapy: an advanced and precise radiotherapy
Proton therapy involves treating tumors with accelerated protons. This advanced technique is reserved for cases where tumors are located near critical organs sensitive to radiation, when traditional methods become dangerous or ineffective.
Why protons ? Because the proton path in matter are very different from that of electrons and X-rays used in conventional radiotherapy. Accelerators also make it possible to use other charged particles, such as carbon ions.
Historically, the idea of using particles produced by accelerators against cancer was due in 1946 to the American physicist Robert Wilson. The pioneers of proton therapy used accelerators dedicated to physics, the first at Upsala in Sweden (1957) and Harvard (1961) in the USA. The first installation dedicated to proton therapy was that in 1989 at the Claterbridge Center for Oncology in the United Kingdom.
Although still marginal, proton therapies and more generally hadron therapies have been in full development for two decades. At the end of 2016, 67 centers were operating worldwide, with 168,000 patients treated. In 2021, 130 centers were expected to be in operation.
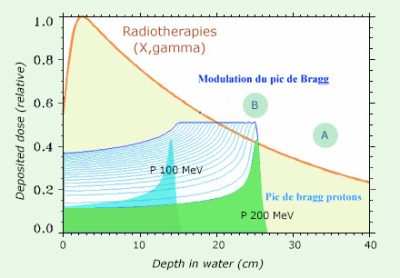
Principle of protontherapy
The figure shows the advantage of using protons instead of X or gamma rays when a sensitive issue (A) is situated behind the tumor (B). For these rays, the absorption is progressive, so that the irradiation also affects the sensitive tissue. On the contrary, protons stop at a certain depth for a given energy and deposit the maximum energy before they stop. Since the protons are delivered by an accelerator, their direction and energy can be adjusted so that the destructive power is maximum in the tumor and spares the sensitive organ located behind.
© IN2P3
Apha particles are efficient weapons for destroying cancer cells because they are very ionizing. They deposit all their energy over a very short distance but their penetrating power is unfortunately very low. Hence the idea of replacing them with more penetrating nuclear particles and providing these particles with sufficient energy so that they can reach deep tumors. One needs to use an accelerator for this. Protons are generally accelerated, hence the name proton therapy.
The energy to be communicated to accelerated protons is much higher than the few MeV of radioactive decays. An energy of 200 MeV is required to penetrate 20 cm into water, a medium whose density is close to that of living matter. The 200 MeV energy is approximately 50 times the energy of the radium alpha rays.
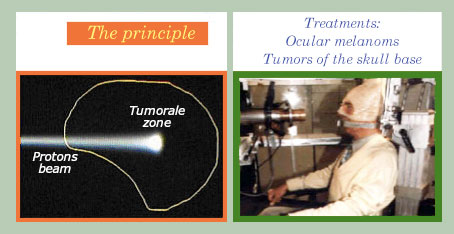
A “conformational” therapy
The diagram on the left shows that protons do not deposit their energy beyond a certain depth. Their energy and direction must be adjusted so that they irradiate mainly the area to be treated. By varying the energies and angles of attack one should match the shape of the tumor. It is necessary, given the ballistic precision, that the patient remains absolutely still (on the right). Precise and fixed marks allow to increase the dose where necessary and reduce it in the healthy tissues.
© Centre de protonthérapie d’Orsay
Having an accelerator gives a ballistic advantage, the possibility of precisely directing the particles onto the tumor, whereas if one relies on the coincidences of radioactive disintegration the rays are emitted in all directions.
Protons deposit the maximum energy at the end of their journey. When a proton enters the body, the energy deposited by ionization initially increases slowly as it slows down, but increases greatly just before stopping. This phenomenon, where the dose delivered is high, is called the Bragg peak. For a therapy, it is a matter of ensuring that as much damage as possible occurs in the tumor.
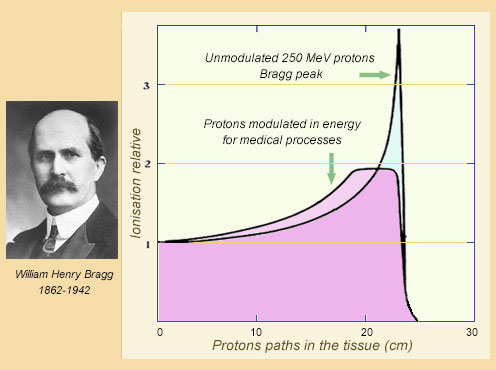
The Bragg curve
The property of particles like protons to lose much energy just before stopping was demonstrated by the English physicist William Bragg. The energy deposited in a tissue by ionization at the end of its path is almost 3 times greater than at the beginning. The path – almost rectilinear – varies little from one proton to another. It is approximately 25 cm for an energy of 250 MeV. By varying the energy of the protons, one reduces the height of the Bragg peak but increases the thickness of the slice where the irradiation is stronger.
© IN2P3
One of the main proton therapy indications is choroidal melanoma, a very serious disease which previously required enucleation of the eye and for which conventional radiotherapy could not be used due to the proximity of the pituitary gland.
For base of the skull tumors resistant to radiation (chondromas and chondrosarcomas), proton therapy allows the dose to be increased in the tumor while sparing critical areas such as the optic nerves, the brain stem, the inner ears or the spinal cord.
The most impressive results were obtained for melanomas of the eye. Nearly 96% of 2,500 patients treated at the Orsay Proton Thérapy Center had not had any recurrences 5 years later and kept their eyes intact. For intracranial treatments, 400 adults were treated in parallel in Orsay. Proton therapy combined with conventional radiotherapy makes it possible to increase tumor control at 5 years to 85-90%, whereas chondromas of the base of the skull lead to death in 2 to 3 years due to the fragility of the encephalic neurological elements.
Given its high technicality, the heavy equipment it requires and its cost, proton therapy remains a tailor-made medicine. For instance, 540 patients were treated in 2003 compared to 8,000 and 170,000 patients treated by brachytherapy and radiotherapy.
In France, there are two main proton therapy centers, that of Orsay and the Antoine Lacassagne Center in Nice. Other hadrontherapy centers have been created in Caen, Lyon and Toulouse.
After a complete renovation in 2010, the Orsay proton therapy center was able to accommodate 550 patients per year, including at least 120 children. The renovated center was equipped with a latest generation accelerator – a cyclotron – and a new treatment room with an isocentric arm, a device allowing the axis of the beam to be oriented in relation to the patient.
Since its opening in 1991, the Antoine Lacassagne center in Nice had treated more than 5,500 patients for eye tumors in 2016. A new proton therapy facility was inaugurated on June 30, 2016 to treat all types of tumors located deep inside the human body.
Initially developed for eye tumors and intercranial tumors, proton therapy is seeing a considerable expansion of its indications, particularly in pediatrics due to the reduction in the risk of treatment-related after-effects.
See also :
Charged particles effects
Ionisation phenomenon
Centre de protonthérapie d’Orsay
Other articles on the subject « Radiation Therapy »
Radiotherapies
X-rays but no radioactivity Radiotherapy today denotes all cancer treatments based on ionizing ra[...]
Metabolic Therapies
Therapeutic Radioisotopes Applications of radioisotopes in nuclear medicine are not limited to sc[...]
Brachytherapies
The oldest nuclear therapy modernized today Brachytherapies (or Curietherapies) are the oldest th[...]
Prostate Brachytherapy
An Efficient destruction of cancerous cells Since the 2000s, particularly in the United States an[...]
Secondary Risks
Alternative: exposure to radiation or let the cancer evolve… For over 20 years, there has b[...]