Doses in Nuclear therapies
High local doses
In nuclear therapy, the doses delivered to tumours amount to few tens of Grays. Like in ordinary radiotherapies, they are considerable because ithey should of destroy malignant cells. The objective remains the same even if the mode of radiation delivery is different: achieve the therapeutic goal by minimizing damages to healthy tissues.
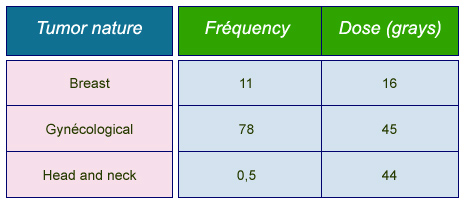
Frequencies and doses in brachytherapies
Average annual frequencies of most common brachytherapies and typical prescribed doses. Frequencies are given per million inhabitants for « Group 1 » countries benefiting from the most advanced care systems (Health Care I countries).
© IN2P3 (Source : K.G. Gerber/Unscear 2000).
One has to distinguish between metabolic therapy – the most practiced – and brachytherapy. In both cases, the exposures are internal.
A characteristic of brachytherapy is that the doses are concentrated near the tumor, where the radioactive sources are introduced. For instance, in breast brachytherapy, beta rays will deposit their energy in the immediate vicinity of iridium-192 wires. Brachytherapy will be preferred if the tumor is well localized. Radiation therapy (X-rays) will be preferred if the tumor is diffuse.
In the case of metabolic therapy – the most frequent beeing that of the thyroid by iodine-131 – the radiopharmaceutical vector is chosen to preferentially attach to the tumor owwing to the chemical affinity of the thyroid for iodine. The beta rays will be again the main source of the dose delivered to the tumour.
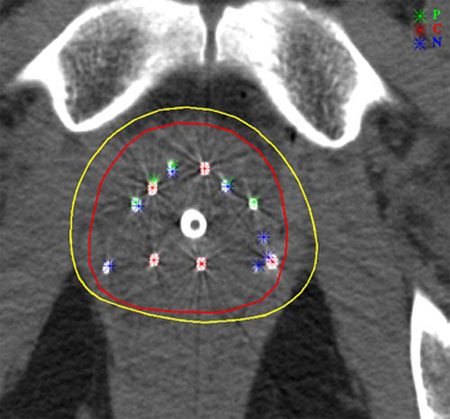
Implants for prostate cancers
Prostate brachytherapy uses implants of iodine-123 or palladium-123 iresponsible for intense local irradiation of the order of 100-150 Gy (grays). This irradiation is very localized and decreases with time time. Witin the yellow line (160 Gy isodose), the energy deposition density exceeds 160 grays. This zone of strong irradiation closely follows the contour of the prostate (line in red).
© Kimmel Cancer Center at Jefferson
The administration of a radioactive isotope for the treatment of a cancer is generally done in a single injection of a high dose of activity of radiopharmaceutical product. The radioactive and biological periods of the radioisotope are relatively short in order to guarantee the relatively rapid disappearance of the radioactive element in the patient’s body. Since the doses of activity are very high, they need to be evaluated accurately so that the risk remains acceptable.
Absorbed doses received are evaluated through complex calculations such as MIRD (Medical Internal Radiation Dose) or simplified dosimetric methods taking into account age, sex and other various factors.
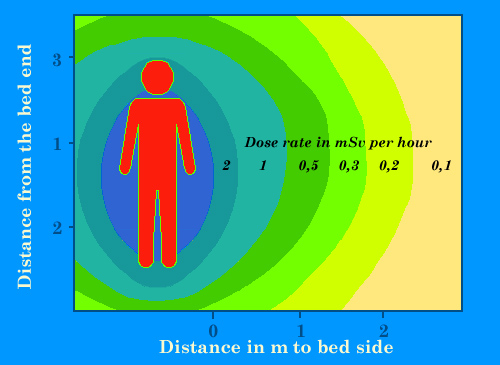
Isolation of patients
Approximate dose rates around the bed of a patient to whom an high dose of 3700 MBq of cesium-137 or 5500 MBq of iodine-131 has been injected for a therapy. Such high doses, commons for instance with brachytherapy or metabolic therapy, require protected rooms for patients hospitalization. These dose rates will decrease within a few days by natural elimination or radioactive decay. Gamma rays will felt near in the room. These therapies requires patients isolation and short intervention of the medical staff.
© IN2P3 (Source : K.G. Gerber).
The risk of administration high activites of radioactive isotopes to patients should be reduced for the medical personnels involved.
Gamma rays that come with most radioactive decays are much more penetrating than beta rays. These gamma irradiate also the medical staff present all year long. When a high dose is injected, for example 5000 Mbq (0.135 millicuria) of iodine-131 for the treatment of hyperthyroidism, an chamber with shielding is necessary where the time of presence of hospital personnel is strictly limited and the wearing of dosimeters mandatory .
The elimination of the iodine takes place in two ways. The 30% that have settled in the thyroid are eliminated with a biological period of about 13 hours. The remaining 70% distributed in the body outside the thyroid is mostly eliminated by the renal route, through urine.
Other articles on the subject « Expositions in Medicine »
Radiation doses in diagnostics
From simple radiology to PET scans and scintigraphy Scintigraphy scans are by far the most common[...]
Doses (X-Rays, CT scans)
Exposures to radiations in diagnostic radiology The vast majority of diagnoses based on radiation[...]
Doses (nuclear diagnostics)
Nuclear medicine : doses in nuclear diagnostics Nuclear medicine examinations are performed only [...]
Doses with therapies
Radio and nuclear therapies: Doses are high but local Genuine nuclear therapies (brachytherapy, p[...]