Transmuting long-lived actinides and fission products
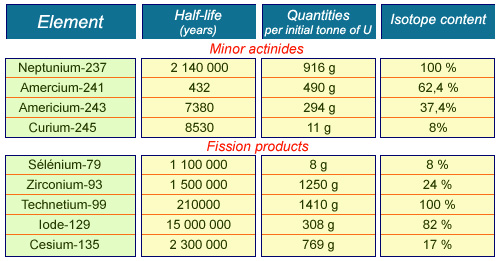
Candidate elements for transmutation
Radioactive elements candidates for transmutation because of their long half-life are the minor actinides and some fission products. The minor actinides are more radiotoxic but are not very mobile. Fission products, on the other hand, are less radiotoxic and can be found in mobile forms such as technetium-99, caesium-135 and iodine-129. The last column shows the content in nuclear waste of the isotope to be transmuted, e.g. caesium-135 for caesium.
© IN2P3
Long-lived elements: actinides and fission products
The radioactive elements selected for transmutation are those that, measured by their half-life, would last for thousands or even millions of years. The advantage of elements being extremely long-lived – their slow activity – is outweighed by the disadvantage of them taking almost an eternity to disappear.
We will look first of all at transmutation of the minor actinides, heavier nuclei than uranium formed in reactors by the capture of one or more neutrons in succession. These nuclei are generally emitters of alpha rays and are radiotoxic if ingested.
Luckily the minor actinides are not very mobile and not very abundant (less than 1 kg per tonne of uranium removed from reactors). They are americium-243 and 241 (with half-lives of 7370 and 432 years respectively), curium-245 (8,500 years) and neptunium-237 (2,100,000 years). Plutonium (a major actinide) does not appear in this list because it is already transmuted in some reactors.
Apart from the actinides, it is worthwhile transmuting the few very long-lived fission products. Fission products, which emit beta rays, are much less toxic if ingested than the actinides. But in some chemical forms, they can be relatively mobile under disposal conditions. This category includes iodine-129 (a half-life of 17,000,000 years), caesium-135 (2,300,000 years) and technetium-99 (213,000 years).
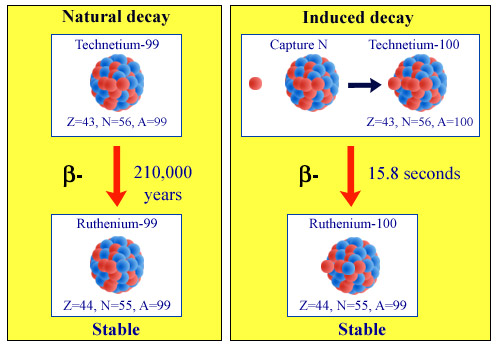
Principle of technetium-99 transmutation
A long-lived fission product – its half-life is 210,000 years – technetium-99 is one of the elements for which transmutation could be envisaged. Capturing a neutron accelerates its return to stability, causing a much more rapid beta disintegration. Once it has captured a neutron, technetium-99 becomes technetium-100, a much more unstable nucleus, which turns into a stable nucleus of ruthenium-100 after a few seconds. Once again, sufficient numbers of neutrons have to be captured to benefit from this phenomenon.
© IN2P3
Technetium-99 is the most abundant element (810 g per tonne of uranium (TU) in nuclear fuel) and the most important one to transmute. Its activity (0.5 Tbq /TU) and its radiotoxicity (175 Sv/TU) account for 96% and 60 % of the total for the three elements. To give an idea of the radioactive risk, how many deaths would be caused by ingesting all the technetium in a tonne of spent fuel? By applying the usual calculation for the linear no-threshold relationship recommended by the ICRP, it would cause 9 deaths. But if the technetium were buried very deep down, only a tiny fraction would end up on the plates of our distant descendants.
/TU) and its radiotoxicity (175 Sv/TU) account for 96% and 60 % of the total for the three elements. To give an idea of the radioactive risk, how many deaths would be caused by ingesting all the technetium in a tonne of spent fuel? By applying the usual calculation for the linear no-threshold relationship recommended by the ICRP, it would cause 9 deaths. But if the technetium were buried very deep down, only a tiny fraction would end up on the plates of our distant descendants.
However, if the use of nuclear continues, the challenge of reducing the quantity of technetium produced is justified. Through the capture of one neutron, the nucleus becomes stable again in a few minutes. Technetium is able to capture neutrons effectively under certain conditions, so the transmutation of this fission product is feasible, if not economical, in specialised reactors.
Caesium-135 (360 g/TU) is not worth transmuting. It represents only 17% of the caesium found in waste. Some of this caesium is stable. Caesium-137 with a 30-year half-life, which is also present, is 80,000 times more active than caesium-135. Irradiation with neutrons would reduce the life of some caesium-135 nuclei but would make radioactive other nuclei that were no longer radioactive. The benefit of doing this is dubious. To make it work, it would first be necessary to separate out the isotope caesium-135, but this would be very expensive to do.
The third element, iodine-129 is also not worth transmuting. This fission product is the least abundant (170 g/TU), its half-life is 17 million years and its activity is only one five-hundredth of that of technetium. A specialist has written that the thyroid can absorb a lot of iodine-129 without it causing any damage, which is a pointed illustration of the fact that, simply because of their different half-lives, iodine-129 is one billion times less active than the iodine-131 dispersed at Chernobyl.
(NB: Iodine-129, which is not properly trapped during reprocessing, is one of the elements that are allowed to be discharged).
Other articles on the subject « Waste strategies »
Slow disappearances
Trapping radioactivity until it disappears A scenario for the long-term future of the waste It wi[...]
Oklo : a natural reactor
Nature at work over the last two billion years It was noticed by chance in the 1970s that a urani[...]
Diluting radioactivity
A practice for elements with low levels of toxicity Management of radioactive waste generally foc[...]
Putting it out of reach
Containment and burial when it is not enough to wait Letting time do its work is not sufficient o[...]
Conditioning
Packages for trapping radioactivity… Conditioning means containing the radioactivity and im[...]
Temporary storages …
Storing: a useful but temporary solution Storing means tidying an object away with the intention [...]
Deep geological disposal
Ground-level and deep disposal: a definitive solution Deep disposal of the most radioactive waste[...]
Separating and sorting
Sorting radioactive waste has advantages As with household waste, sorting can prove really useful[...]
Destroy (transmutation)
Transforming radioactive nuclei to make them less troublesome… The incineration of househol[...]
Transmuting actinides
Attractive in principle, difficult in practice Destroying actinides using fission reactions is at[...]