Neptunium, americium and curium
Minor actinides constitute a very small minority of high activity nuclear waste. around 600 grams per ton of irradiated fuel. A one gigawatt nuclear reactor generates about 17.5 kg per year, which correspond, for all the 60 French reactors, to an annual production of 1.05 ton. If it were not radioactive, all this production would fit into a 50 liters bag ….
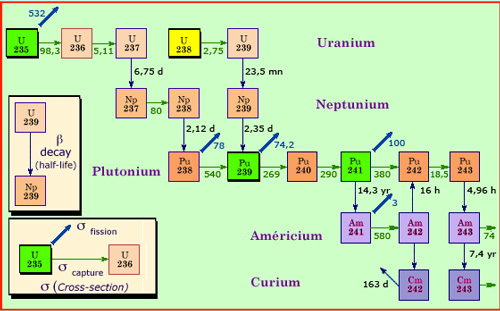
From uranium to actinides
The successive captures of several neutrons lead to the formation of transuranic nuclei, beyond uranium. One can see on this nuclei map, how multiple captures convert uranium-235 and 238 from the fuel into isotopes of plutonium, neptunium, americium and curium, known as actinides. Neutron captures are not frequent. In a fuel that stays 3 or 4 years in a reactor, only 2.5% of uranium-238 nuclei undergo at least one capture. An unstable nucleus resulting from a capture has the time to be transformed by beta decay well before the next capture. If capture leads to a fission, there will be no actinides.
© IN2P3
Neptunium-237 is the most abundant minor actinide. It is generated by neutron captures in uranium-235 nuclei, but it is by far the least active, its half-life or period being of 2.15 million years. It is difficult to destroy, except in reactors with high neutron flux. In such reactors, neptunium-237 would be incinerated in two stages: a first neutron would weaken the nucleus before a second capture destroy it by fission.
The two isotopes of americium (241 and 242) are more radioactive than neptunium-237. The activity of americium-241, whose period is of 432 years, becomes predominant at the century time scale. This isotope accumulates in the nuclear spent fuel, because fresh americium-242 comes from the decay of plutonium-241, which lives only 14 years.
Because of its short lifetime (18 years), curium-244 is highly radioactive, but it disappears at the scale of a century. It contributes to about 60% of the initial radioactivity of minor actinides, although it is produced in very small quantities.
When plutonium is incorporated in the nuclear fuel, less neutron captures are required for the synthesis of minor actinides which have more than 240 nucleons. That is why the formation of americium is quintupled with MOX fuel. This increased production of minor actinides is one major shortcoming of this fuel.
Compared to the isotopes of plutonium, minor actinides contribute much less in terms of activity and toxicity in the spent fuel. Initially, they represent only 2.5% of its radioactive activity; the rest being due to plutonium whose isotope 241 with its 14-year half-life is very active. Therefore, if plutonium is extracted from the spent fuel to be burned later on, the waste radioactivity and toxicity are much reduced.
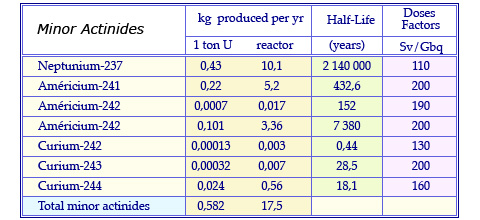
Characteristics of minor actinides
Quantities of minor actinides produced annually in the fuel of a conventional pressurized water reactor. The fuel has undergone a standard combustion (burn-up) of 33 Gigawatt per day and was retrieved after 3 years in the reactor core. These quantities are quoted for 1 ton and 23 tons, the annual consumption of uranium. The table quotes also the half-life of these elements and their dose factor that estimates their potential toxicity.
© IN2P3
In France, minor actinides are packaged with fission products in the vitrified waste generated by the reprocessing of spent fuel. When short and medium half-life fission products have vanished, the contribution of actinides to the waste radioactivity becomes dominant. The high activity vitrified waste from reprocessing is then 7 times less radioactive than spent fuel in which plutonium has not been extracted. When or if a future improved reprocessing is able to separate minor actinides in order to burn them (for example, in breeders or hybrid reactors), it would become possible to go much further in reducing the waste radioactivity.
Other articles on the subject « Nuclear Fission »
200 millions electronvolts !
A huge amount of energy at the atomic scale The fission of a uranium or plutonium nucleus liberat[...]
Chain Reaction
From one single fission to fissions on a massive scale Nuclear fission emits a lot of energy on t[...]
Fissile nuclei
Uranium 235 and 233, plutonium 239 The few fissile nuclei found in nature belong to heavy atoms, [...]
Fission fragment
Chronicle of the life of a fission fragment … The fission of a nucleus into two fragments i[...]
Fission products
The ashes of nuclear fission Fission products are the remains of a heavy uranium or plutonium nuc[...]
Plutonium-239 formation
Transforming a fertile nucleus into a fissile nucleus Uranium-238 accounts for more than 95% of t[...]
Short-lived Fission Products
Most fission products vanish rather rapidly The vast majority of the radioactive fission products[...]
Long-lived Fission Products
A handful of tough but little radioactive isotopes … The more a nucleus is long-lived, the [...]
Actinides
A class of very heavy radioactive nuclei Alongside the fission products found in the core of nucl[...]
Plutonium Isotopes
Plutonium-239 but also 238, 240, 241, 242 … Plutonium isotopes are produced by neutron capt[...]