Attractive in principle, difficult in practice
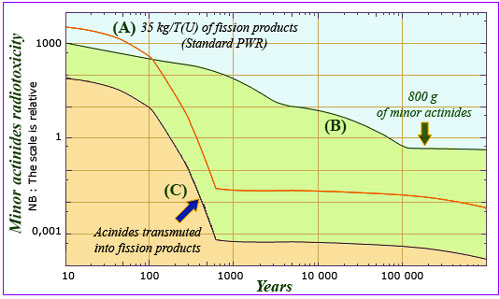
A large reduction in the radioactive toxicity of waste…
The toxicity reduction obtained by transmuting minor actinides into fission products is large(green area). The actinides are transmuted into much more short-lived fission products (curve C). The curve is obtained by dividing the contribution of the fission products to the spent fuel of a PWR reactor (curve A) by the ratio of the fissile masses: 35 kg per tonne of uranium on the one hand and 800 g of actinides to undergo fission on the other. In principle this reduction is of several orders of magnitude.
© IN2P3
Destroying actinides using fission reactions is attractive on paper but difficult in practice. The principle is as follows: the capture of a neutron by the fragile nuclei that are actinides often but not always causes them to fragment. The two fragments become stable again much more quickly than the nucleus they came from.
The nucleus emitted alpha rays, but the fragments of it emit much less toxic beta rays. Transmutation not only speeds up the return to stability, but also reduces radiotoxicity.
The very slow decay of the actinides explains why more trouble is taken over them than over the fission products present in much larger quantities in the spent fuel from reactors (35 kg per tonne of uranium compared with 800 g, under the normal operating conditions of a PWR). Apart from curium-244 (which has a half-life of 18 years), their half-lives range from 432 years for americium-241 to 2.1 million years for neptunium-237. The fission products decay much more quickly.
The vast majority of fission products are short-lived. After thirty years, only a handful are still radioactive: two nuclei, caesium-137 and strontium-90, dominate the radioactive decay process for the next 600 years, at the end of which only a few very long-lived fission products, like technetium-99, remain.
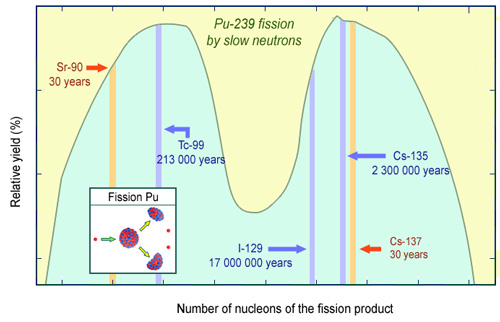
Distribution of the plutonium fission products
The distribution of the number of nucleons of the fission products of a minor actinide is similar to that of a fissile heavy nucleus such as uranium-235 or plutonium-239. Almost all the fragments from fission, initially highly radioactive, become stable again after a few years. Some of the fission products shown in the figure persist: strontium-90 and caesium-137 with a half-life of around 30 years; and technetium-99, iodine-129 and caesium-135, with half-lives above hundreds of thousands of years.
© IN2P3
Fission of the actinides produces fission products similar to those produced by the fission of uranium and plutonium. The proportions of the handful of nuclei that determine the speed of radioactive decay are very similar. The radiotoxicity of transmuted actinides can be estimated fairly accurately by dividing the radiotoxicity of the fission products by the ratio of their masses (35 kg and 800 g). This simple calculation shows that the fission of a minor actinide reduces the radiotoxicity by a factor of 10 after 10 years, 200 after 300 years and 100,000 after 600 years.
The reality is not as glorious as these figures suggest. The fission of significant quantities of actinides is not an easy task. Fission is a nuclear reaction that means they have to go back into the reactor. In what form should the actinides be incorporated into the nuclear fuel? This incorporation is not without its risks: if too much is incorporated, it disrupts the operation of the reactor and causes instability.
Secondly, fission has to compete against simple neutron capture, which makes the nucleus fragile but does not destroy it. The probability of fission occurring varies widely from one actinide to another and also varies with the energy of the neutrons. It is generally better to use fast neutrons than slow neutrons. Even with fast neutrons, several captures are often needed to break a nucleus, just like several blows of an axe are needed to split a difficult log …
Lastly, the probabilities of neutron capture (particularly with fast neutrons) are quite low, and the number of nuclei to be transmuted is very large, if we think about Avogadro’s number. Very large neutron flows are needed to destroy a few kilogrammes of actinides. It is also necessary not to produce more actinides in the rest of the fuel than you are destroying, which will only be possible with reactors specially designed for this. Even with such reactors, achieving a high transmutation efficiency will be difficult. We are still only at the experimental stage.
Other articles on the subject « Waste strategies »
Slow disappearances
Trapping radioactivity until it disappears A scenario for the long-term future of the waste It wi[...]
Oklo : a natural reactor
Nature at work over the last two billion years It was noticed by chance in the 1970s that a urani[...]
Diluting radioactivity
A practice for elements with low levels of toxicity Management of radioactive waste generally foc[...]
Putting it out of reach
Containment and burial when it is not enough to wait Letting time do its work is not sufficient o[...]
Conditioning
Packages for trapping radioactivity… Conditioning means containing the radioactivity and im[...]
Temporary storages …
Storing: a useful but temporary solution Storing means tidying an object away with the intention [...]
Deep geological disposal
Ground-level and deep disposal: a definitive solution Deep disposal of the most radioactive waste[...]
Separating and sorting
Sorting radioactive waste has advantages As with household waste, sorting can prove really useful[...]
Destroy (transmutation)
Transforming radioactive nuclei to make them less troublesome… The incineration of househol[...]
What to transmute?
Transmuting long-lived actinides and fission products Long-lived elements: actinides and fission [...]