A risk indicator more pessimistic than radioactive activity
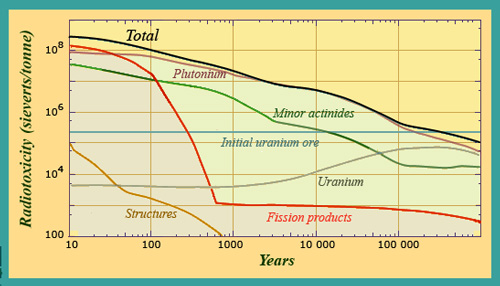
Spent fuel radiotoxicity evolution from 10 to 1 million years
The pattern of change in the radiotoxicity of spent fuel highlights the predominance of plutonium. This element overtakes fission products around 50 years after removal from the reactor.
© Source: CEA
Radioactivity is an imperfect descriptor of radioactive risks. It makes no allowance for the major difference in harmfulness between radioactive atoms, or for the nature of the emitted radiation and the exposure conditions.
For example, alpha rays from plutonium contain approximately 1,000 times more energy than beta rays from tritium. Alpha rays are highly toxic to living matter. However, alpha rays are blocked by the thickness of a sheet of paper, and are totally harmless in case of external exposure.
How to realistically assess risk? In principle, all reasonable precautions are taken to ensure that radioactive atoms in spent fuel assemblies and radioactive waste packages remains trapped inside them forever, or at least until they become harmless. They should almost never come into contact with living tissue and the most highly-penetrative rays will not reach us after waste is buried.
The danger relates to the tiny minority of radioactive atoms that may migrate from the waste into the biosphere, contaminating it and presenting the risk of ingestion or inhalation by our distant descendants. The risk posed by a radioelement depends on the atom’s mobility and toxicity. Plutonium and minor actinides, which are generally alpha emitters, are by far the most toxic; fortunately, these heavy nuclei are very immobile. Fission products are more mobile, in particular in species such as iodine and caesium, and to a lesser extent technetium.
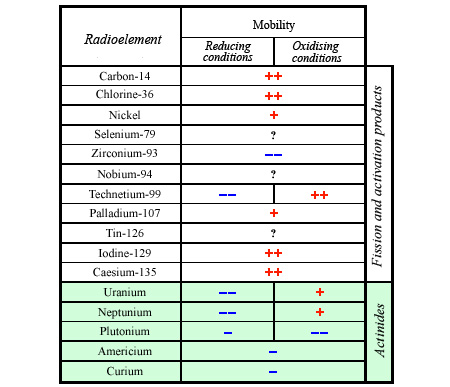
Mobility of some radioelements
When assessing the actual risk, it is important to consider the mobility of the radioactive species mobility in the environment. This requirement does not apply to « radiotoxicity ». The table shows that the relative mobility of radioelements varies according to whether the host environmental conditions are oxidising or reducing. The ++ and + signs indicate high mobility, and — and – signs indicate low mobility.
© CNE (French National Evaluation Commission )
As it is not possible to predict the quantity of radioactive atoms ingested in the distant future, physicists and engineers adopt the worst-case assumption that all such atoms are ingested. They work with a potential radiotoxicity indicator.
The decrease in potential radiotoxicity over time mirrors the decrease in activity. It reflects the change in risk in case of ingestion of the most radiotoxic elements: plutonium and minor actinides. However, this risk should not be confused with the actual risk, as it does not take the mobility of the relevant radioelements into account.
Vitrified waste from which the plutonium has been removed will consequently be less radiotoxic than untreated fuel. This is one of the arguments in favour of reprocessing, subject to reusing the plutonium elsewhere. Fuels may also be compared from a radiotoxicity perspective. For example, spent MOX fuel will be more toxic than spent uranium fuel, as it contains more plutonium and minor actinides.
Other articles on the subject « Spent nuclear fuel »
Reactor unloading
Fuel unloading and initial pool storage of spent fuel The fuel load in a conventional pressurised[...]
Spent fuel Burn-up
Irradiation rate and energy supplied by fuel The energy produced by a nuclear power plant is prop[...]
Spent fuel composition
Key figures on spent fuel composition The composition of irradiated fuel removed from a reactor c[...]
Waste radioactivity decrease
A natural but slow decay process As natural radioactivity progressively decays, the radioactivity[...]
Spent fuel decay heat
Significant, slow-to-decline heat release The heat released by radioactive disintegration is a ke[...]