Development of processes and extractant molecules
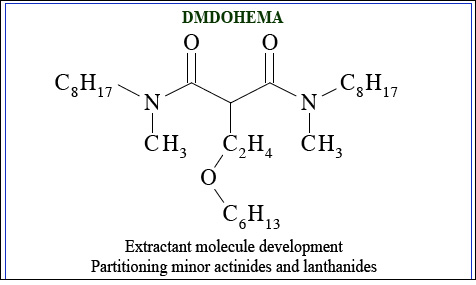
Extractant molecule development: DMDOHEMA
Extractant molecules have been chosen for various stages of actinides partitioning. One of them is DMDOHEMA, which has the advantage once it has decayed of being easy to burn without leaving mineral waste. This organic molecule can extract americium, curium and the lanthanides together. For the next stage – partitioning of americium and curium from the lanthanides – other organic molecules (HDEHP and HEDTA) are used.
© CEA
The 2006 assessment of research into partitioning was promising given that this was an area that was almost entirely unexplored at the outset. Efforts have focused mainly on the partitioning of the minor actinides in aqueous nitric acid solutions. This method, referred to as hydrometallurgical, is currently considered the reference for some ambitious but less well developed alternatives, such as group partitioning or pyrochemical processes.
Processes have been developed for partitioning the minor actinides and certain fission products in solution, mainly in facilities at the Atalante laboratory in Marcoule. CEA’s chemists have found some selective and strong extractant molecules, in particular for americium and curium and for extracting fission products like caesium.
The scientific feasibility of partitioning neptunium (the most abundant but least radioactive minor actinide) by adapting the PUREX industrial process was demonstrated as far back as 1995, and the technical feasibility in 2003. The partitioning of americium and curium, which is more difficult, requires joint extraction with the lanthanides as a first step, followed by partitioning from the lanthanides.
The scientific feasibility of the joint extraction of the actinides and lanthanides using the DIAMEX process was established in 1994-1995. Research led to the adoption in 1998 of a complex molecule, DMDOHEMA, to jointly partition these radioelements from the other fission products. The efficiency achieved is around 99.9% for the actinides.
For the next step (SANEX), extracting the actinides from the lanthanides, two avenues have been explored. The first was to use extractants that are effective in a very acid medium, the bis-triazinyl-pyridines (BTBPs), but these proved to be unstable. The second was based on a combination of two extractants, a diamide capable of extracting actinides and lanthanides from a very acid medium (HDEHP) and an organic acid capable of extracting these ions from a medium of low acidity (HEDTA). This combination has been successfully tested in the laboratory and on a larger scale.
Demonstration of the selective recovery of the minor actinides was achieved at the end of 2005 with an experiment in Atalante on around ten kilos of fuel instead of the few hundreds of grams previously processed. An intermediate scale like this is still a long way from an industrial process, but the 2005 tests showed that with the right combination of extractants (DMDOHEMA, HDEHP, HEDTA), 99.9% of the americium and 99.7% of the curium could be recovered, with a partitioning factor of actinides from lanthanides of 800.
Caesium partitioning: the calixarene-crowns
The calixarene-crowns, molecules developed at CEA, have a particular affinity for caesium. Caesium partitioning is carried out after the partitioning of the actinides and lanthanides in the DIAMEX process. Caesium-137, which accounts for 44% of the caesium in spent fuel, makes a major contribution to the heat released by the waste to be buried, but after 300 years has disappeared. Extracting the caesium from this radioactive waste and conditioning it separately would reduce the size and cost of deep geological disposal facilities.
© CEA
Fission product partitioning
Caesium partitioning could prove useful when it comes to waste disposal since one of the isotopes concerned, caesium-137, makes a major contribution to the heat released initially but little contribution after 100 years.
Chemists have developed some amazing molecules, the calixarene-crowns, which have a particular affinity for caesium. These molecules were developed notably by CEA in 2001-2002. All that remains is to develop a partitioning process using these calixarenes and to develop its technical feasibility.
in 2001-2002. All that remains is to develop a partitioning process using these calixarenes and to develop its technical feasibility.
For the other fission products that make suitable candidates for partitioning, studies have shown that by adapting the PUREX process, 99% of the iodine and 90% of the technetium could be recovered. The goal is to master the process of partitioning iodine, which has now been achieved with 95% efficiency on an industrial scale. Then the recovery of the technetium needs to be increased to 99%, above the 90% figure already achieved with the soluble fraction.
Other articles on the subject « Waste Outlooks »
Three research areas
Three complementary research areas The three research areas under the Bataille Act, later supplem[...]
Area 1: Partitioning
Sorting the radioelements in radioactive waste The separation, or partitioning, of the radioactiv[...]
Advanced partitioning
Partitioning of nuclear waste: hot chemistry Advanced partitioning goes beyond just the partition[...]
Area 1: Transmutation
Area 1: Transmuting or transforming radioactive atoms Transmutation is the second part of researc[...]
Transmutation facilities
Fast reactors and Accelerator Driven Systems (ADS) The neutron, which easily penetrates nuclei, i[...]
Transmutation prospects
A far away objective for Generation IV and 2040… At the time of the first tests on the tran[...]
Area 2: Disposal
Research into deep geological disposal Can the nuclear industry’s final waste, which we cur[...]
Area 3: Long Surface Storage
Research into long-term storage Research into long-term storage is the second research topic in a[...]
Long surface storage concepts
Long-term surface and subsurface storage Research into very long-term storage is a matter for the[...]
Area 3: Conditioning
Area 3: Research into conditioning The conditioning of high-level and intermediate-level waste is[...]