Leaving be and giving it time
Successive periods of dominance…
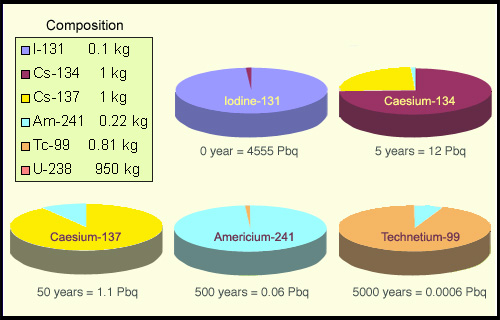
Simplified waste made of six main radioelements present in the spent fuel of a reactor. The ‘pies’ show the contribution of the six elements to the activity of this waste at times 0 and 5, 50, 500 and 5000 years later. Each of these times is characterised by the dominance of one particular element. Initially, iodine-131 is the dominant element. After 5 years, the iodine-131 has disappeared and caesium-134 is dominant, and so on until technetium takes its turn after 5000 years. In the meantime, the activity has decayed considerably. It will be millions of years before the uranium becomes the dominant element, despite the fact that it accounts for almost all of the mass.
© IN2P3
Give it time was one of François Mitterrand’s favourite maxims. Far from the cut and thrust of politics, this maxim really does have to be followed when it comes to managing radioactive waste.
When a radioactive nucleus emits radiation, it loses some of its radioactivity. A fission product, for example, turns into a stable nucleus. The shorter the half-life of a radioactive nucleus, the quicker it becomes stable. After around ten half-lives the radiation emitted is reduced by a factor of 1000. If we can wait that long or longer, this is how much the radiological risk from this radiation will have decreased.
The most active elements are the ones that disappear quickest. A good illustration of this is given by the example of four radioelements representative of the spent fuel from reactors. These radioactive nuclei, which have very different half-lives, are iodine-131 (half-life of 8 days), caesium-137 (30 years), technetium-99 (210,000 years) and the actinide americium-241 (433 years). Because of the disparity of these half-lives, iodine-131 is 1300 times more radioactive than caesium-137 for an equal number of atoms. Caesium-137 is 14 times more active than americium-241, which is itself 485 times more active than technetium-99.
Because of its extremely high level of activity, a few months after the reactor is stopped the iodine-131 will have completely disappeared. On the other hand the caesium-137, an abundant fission product, will have time to pass through a reprocessing plant and be contained in a glass matrix, though our distant descendants would find that it had disappeared from the glass package within a few centuries. An actinide like americium-241 will take several millennia to disappear. In principle it should remain captive in the glass for the whole of this time.
Finally, with the technetium, which has a half-life of 210,000 years, we can no longer count on the glass being intact. But this long half-life is offset by its low level of activity: in 100 years only one technetium atom decays among 3300!
Letting time do its work is good for the most radioactive elements with short or medium half-lives. That is why spent fuel assemblies are stored in the pools of reactors and reprocessing plants, and why vitrified waste is stored in ventilated shafts until a decision is made about its fate.
NEXT : A race to be slowest
NEXT : Oklo
Other articles on the subject « Waste strategies »
Oklo : a natural reactor
Nature at work over the last two billion years It was noticed by chance in the 1970s that a urani[...]
Diluting radioactivity
A practice for elements with low levels of toxicity Management of radioactive waste generally foc[...]
Putting it out of reach
Containment and burial when it is not enough to wait Letting time do its work is not sufficient o[...]
Conditioning
Packages for trapping radioactivity… Conditioning means containing the radioactivity and im[...]
Temporary storages …
Storing: a useful but temporary solution Storing means tidying an object away with the intention [...]
Deep geological disposal
Ground-level and deep disposal: a definitive solution Deep disposal of the most radioactive waste[...]
Separating and sorting
Sorting radioactive waste has advantages As with household waste, sorting can prove really useful[...]
Destroy (transmutation)
Transforming radioactive nuclei to make them less troublesome… The incineration of househol[...]
What to transmute?
Transmuting long-lived actinides and fission products Long-lived elements: actinides and fission [...]
Transmuting actinides
Attractive in principle, difficult in practice Destroying actinides using fission reactions is at[...]