A practice for elements with low levels of toxicity
Management of radioactive waste generally focuses on concentrating radioactivity in one place, containing it and burying it as deep as possible. However, sometimes the opposite is done: waste is diluted in a vast volume of water, with the hope that the action of currents and wind will make the dilution uniform. These discharges are now only permitted in the case of radioactive elements with very low levels of toxicity that are difficult, or indeed impossible, to trap.
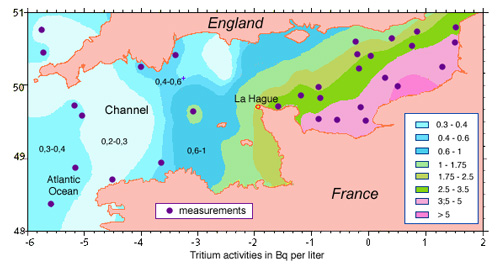
Tritium monitoring in the Channel
Its radioactivity makes tritium an easy radioelement to monitor. This map of the Channel shows that tritium activity due to discharges from La Hague does not exceed 5,500 Bq/m3 despite non uniform dilution. One would have to drink 10,000 m3 a year of the most polluted water to reach the annual regulatory limit of 1 mSv (this is because of tritium’s low radioactive toxicity).
© IN2P3 (source IRSN)
French laws allow the reprocessing plant at La Hague to discharge tritium, which is a difficult element to fix, into the sea. These discharges are permitted because of tritium’s low levels of toxicity: 1 becquerel of tritium is 4000 times less harmful than 1 becquerel of plutonium. A volume of water like that of the Channel is in principle sufficient to bring the tritium activity down to an acceptable level for health.
Tritium is an easy radioelement to monitor. Maps of the Channel show that, although its dilution is not uniform, it is satisfactory. The tritium activity remains below levels that would constitute a risk. The La Hague plant also discharges into the atmosphere krypton-85, a radioactive rare gas, that is chemically inert and therefore impossible to trap. Krypton has no dangerous radioactive descendants like radon. Exposure is external. Furthermore, by a lucky chance krypton only emits penetrating gamma rays one time out of every hundred!
to monitor. Maps of the Channel show that, although its dilution is not uniform, it is satisfactory. The tritium activity remains below levels that would constitute a risk. The La Hague plant also discharges into the atmosphere krypton-85, a radioactive rare gas, that is chemically inert and therefore impossible to trap. Krypton has no dangerous radioactive descendants like radon. Exposure is external. Furthermore, by a lucky chance krypton only emits penetrating gamma rays one time out of every hundred!
In the case of tritium, forthcoming legislation aims to reduce discharges further by immobilising the tritium. However, there is a high cost to dealing with these low-toxicity becquerels. Should it really be a radiation protection priority? For example, it would seem more sensible to invest effort earlier on in the chain, as the medical establishment is advocating, by reducing the doses received during common examinations such as scans.
These permitted tritium and krypton discharges should not be confused with others that have now been stopped. Dilution was the technique used in the early days of civil and military nuclear, at a time when there were much smaller volumes of radioactive material and a less scrupulous approach was taken.
Some discharges continued for a long time. Until 1993, the Soviet (then Russian) naval authorities of the Far East fleet were discharging radioactive fluids, from the reactor vessels of nuclear submarines that were being dismantled, into the Sea of Japan. The main radioactive atoms discharged into the sea were caesium-137 and strontium-90. The activity due to these discharges was not great but these elements are more radiotoxic than tritium and krypton.
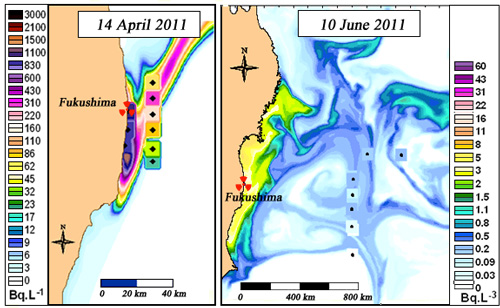
Fukushima: Dilution of radioactivity in the sea
These two maps show the change in caesium-137 concentrations in the sea off Fukushima between April 2011, just after the main discharges, and June 2011. The change in the concentrations reveals effective dilution of the seawater contamination within two months. This dilution is the result of the contaminated waters being carried by strong currents towards the centre of the North Pacific. (NB: This IRSN map was prepared from an Ifremer model).
© IRSN/Ifremer
Quite apart from these rare deliberate discharges, which are banned, dilution contributed to lessening the consequences of the Fukushima accident.
At the start of April 2011, a vast flow of highly contaminated water from reactor No 2 was escaping from a pool linked to the sea. The flow was not stemmed until 5 April, several days later. Caesium-137 concentrations of tens of thousands of becquerels per litre were measured from the plant.
Maps of the concentrations at sea off Fukushima, prepared by IRSN, show that two months later these concentrations had fallen to tens of becquerels per litre. The effect of dilution combined with the strong ocean currents explains this spectacular reduction.
Other articles on the subject « Waste strategies »
Slow disappearances
Trapping radioactivity until it disappears A scenario for the long-term future of the waste It wi[...]
Oklo : a natural reactor
Nature at work over the last two billion years It was noticed by chance in the 1970s that a urani[...]
Putting it out of reach
Containment and burial when it is not enough to wait Letting time do its work is not sufficient o[...]
Conditioning
Packages for trapping radioactivity… Conditioning means containing the radioactivity and im[...]
Temporary storages …
Storing: a useful but temporary solution Storing means tidying an object away with the intention [...]
Deep geological disposal
Ground-level and deep disposal: a definitive solution Deep disposal of the most radioactive waste[...]
Separating and sorting
Sorting radioactive waste has advantages As with household waste, sorting can prove really useful[...]
Destroy (transmutation)
Transforming radioactive nuclei to make them less troublesome… The incineration of househol[...]
What to transmute?
Transmuting long-lived actinides and fission products Long-lived elements: actinides and fission [...]
Transmuting actinides
Attractive in principle, difficult in practice Destroying actinides using fission reactions is at[...]