Nuclear spent fuel and vitrified waste: what legacy?
« Much of this nuclear waste will remain hazardous for hundreds of thousands of years, leaving a poisonous legacy to future generations ». This message from the environmental organization Greenpeace, generates understandable fears. What is its veracity? What do the physicist, the engineer think? What does Nature say?
A 100,000 years time frame is often quoted. Will humanity reach this great age, 50 times the years that have passed since the birth of Christ. At 100,000 years, will there still be humans on Earth? Will they have disappeared or will they have gone to another planet?
Unlike humans, the fate of radioactive atoms is mathematically predictable. Most of them will be gone, but not completely. We are at the very beginning of the path that leads to extraordinarily distant deadlines. In 2015, the oldest waste was barely fifty years old!
100,000 years for the most radioactive waste? … Slogan or reality ?
It is impossible to exactly define a duration for a radioactive species, because mathematically one can not define the moment when the species will disappear. On the other hand one can define the moment when 50%, 90%, 99.9% of its atoms will have disappeared. In a radioactive package, the most active radioelements, therefore the most dangerous, will disappear slowly at the scale of human lives, but well before the first millennium! For instance, cesium-137 will lose 99.9% of its radioactivity in 3 centuries. On the other hand only 3% of the atoms of a minor actinide like neptunium-237 will have decreased in 100 000 years. The species remaining at these time scales are not very radioactive. A neptunium atom is for example 71 000 times less active than a cesium-137 one: If we are lucky enough to reach 100 years, only 1 nucleus of neptunium out of 31000 will have emitted a ray during our earthly stay!
To clarify what is said above, let us compare the evolution of the radioactivity of the two types of waste envisaged for a deep geological repository in France: the spent fuels coming out of the reactors ; the high activity vitrified waste produced at the La Hague plant by reprocessing these spent fuels. In the latter, plutonium and uranium that can be reused have been removed. Based on standard compositions, this comparison gives a good idea of the level of radioactivity of both types of waste and their evolution.
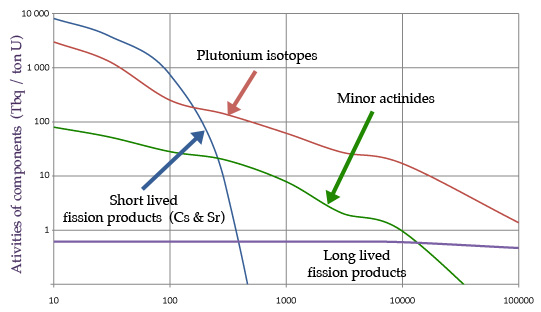
Evolution of activities for one ton of spent fuel
Characteristic evolutions – from 10 years to 100,000 years ago – of the activity of the main elements present in a spent fuel: plutonium, short-lived fission products, minor actinides and long-lived fission products. The activities expressed in terabecquerels are calculated for a fuel initially containing one tonne of uranium and discharged from a reactor after a standard irradiation of 3 years.
IN2P3
Let us first consider a tonne of spent fuel that has undergone standard irradiation. Unloaded from the reactor, it contains four main types of radioactive atoms: plutonium isotopes, short-lived fission products including cesium-137 and strontium-90, minor actinides (neptunium, americium and curium), and a handful of long-lived fission products. (NB: the radioactivity of 940 kg of uranium is negligible)
The radioactivity of the ten kilograms of plutonium is dominant throughout the 100,000 years. The overall radioactivity decreases very slowly due to the very long radioactive half-lives of certain isotopes. But the decay is very important at the scale of one hundred millennia, more than 5,000 times, according to the evaluation of the curve below in the case of spent fuels; more for vitrified waste.
The contribution of two short-lived fission products, cesium-137 and strontium-90, competes with that of plutonium during the first hundred years, the years we are living. But divided by 1,000 every 300 years, it will be the first to vanish. The decay of minor actinides is similar to that of plutonium, also an actinide. Their contribution is 10 to 20 times lower than plutonium. Finally, the radioactivity of the fourth category, the long lived fission products, is very low and its contribution negligible due to extremely long lifetimes.
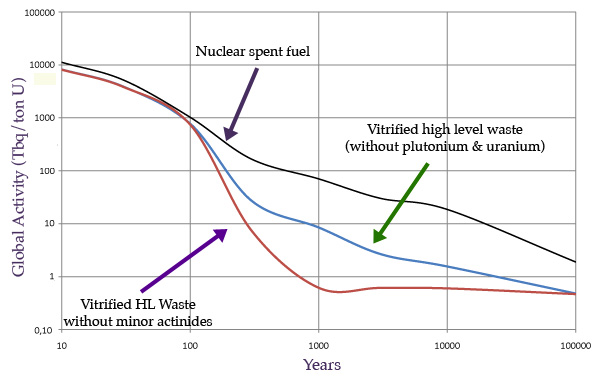
Fig 2 : Decrease of activity in spent fuel and high-level waste
Compared evolutions, evaluated for one ton of initial uranium, of the activities of three types of waste: 1) Standard nuclear spent fuel stored as waste; 2) vitrified waste obtaned from spent fuel with plutonium removed; (3) Vitrified waste from which minor actinides have also been removed. The diminution of radioactive activity in the long term is 10 times faster for the second, and even more so for the third.
© IN2P3
In 100,000 years, the radioactivity will have decreased nearly 10,000 times …
Figure 2 compares the evolution of the radioactivity of spent nuclear fuels and vitrified waste packages produced in France. Their radioactive activity and the risks they presents will have decreased several thousands of times at the end of the 100,000 years. However, this decrease remains very slow. How to accelerate a return to activities if not harmless at least well attenuated?
The figure also shows that removing plutonium from spent fuel divides radioactive activity by 3 to 8 beyond 100 years, and by 1000 at the end of the first millennium. It will take less than 10,000 years to reach the level of decay that nuclear spent fuel will reach in 100,000 years. In addition, the radioactive atoms – fission products and minor actinides – are conditioned at the La Hague plant within a vitreous material, the virtue of which is to immobilize them for millennia. The resilience of these glasses to radiation would be at least 10,000 years, at an age when their radioactivity would be divided by about 5,000.
To further accelerate the diminution of radioactivity, it is planned to remove minor actinides as well. The 100,000 years would then be reduced to around 300 years. But, what to do with plutonium and minor actinides? The plutonium removed is not of a military grade. It can be burned as fuel in fast neutron reactors. As for the minor actinides, less abundant, they could be also burned so if waste burners reactors saw the light some day.
One can not consider storing indefinitely near humans highly radioactive material such as spent fues or vitrified waste. For the former, it will be necessary one day or the other to reprocess them, or to bury them at a great depth, or both at the same time. Some activists oppose both the deep storage of these materials, reprocessing that frozes their radioactivity, after having opposed in France the first reactor plutonium burner (Superphenix). To follow them, to refuse all the solutions, is the best way « to leave to future generations a poisoned legacy« !
Logic and a positive ecology would not oppose techniques aimed at reducing the quantity and activity of long-lived waste that some day or another will require to be stored. Let us remember the wise words of a specialist; « If we want to store the long-lived waste in stable underground geological layers, it is to add a further barrier between the radiations emitted by this waste and us, but it is also to protect the waste against human intrusions, intentionals or not: at 500 meters underground, they will be safe. »
Other articles on the subject « Geological disposal »
CIGEO project
CIGEO – Overview of a planned repository The efforts made by engineers to ensure that highl[...]
Clay medium
The Callovo-Oxfordian argilite The geological stratum or ‘host rock’ that will[...]
Bure underground laboratory
A research laboratory for a disposal facility site Before high-level radioactive waste is buried [...]
Results on deep clay disposal
What we know about the disposal facility site In its final report in 2006 the French National Ass[...]
High-level waste repositories
Disposal of high-level waste packages The high-level waste packages requiring disposal will proba[...]
ILW-LL waste repositories
Filling an intermediate-level waste disposal cell Intermediate-level (ILW-LL) waste releases litt[...]
Waste disposal USA
Back to the drawing board after Yucca Mountain WIPP, the first deep geological repository to begi[...]
WIPP Project
The world’s first deep geological repository The United States is the first country in the [...]
Waste disposal Sweden
Sweden and Finland – Exemplary waste management Sweden probably features among the E[...]
Other countries
Shared challenges and similar solutions In most countries with nuclear industries, solutions for [...]