Find the age of a bygone object by counting Carbon-14 decays
The most common of the radioactive dating techniques currently in use involves the isotope 14 of carbon, the radiocarbon. This radioactive isotope of carbon is present in the atmosphere in trace amounts. In chemical processes carbon 14 is indistinguishable from normal carbon 12. As a result, animal and plant life regularly assimilate carbon 14 atom together with the carbon 12.
The carbon 14 present in the atmosphere is constantly renewed. The cosmic rays originating from the Sun collide with nuclei in the upper atmosphere and are capable of breaking off individual neutrons. These neutrons, once freed, can interact with atoms of nitrogen 14 in air, causing the expulsion of a proton and the formation of carbon 14.
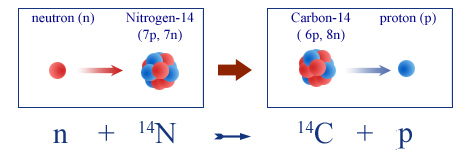
Formation of carbon-14 from atmospheric nitrogen
© IN2P3
One naturally assumes that the cosmic bombardment responsible for this transmutation remains constant over the millennia. The rate of cosmic rays which hit the Earth depends on two very slowly changing factors: the solar activity and the Earth’s magnetic field. This latter serves as a shield against all cosmic radiation – when its strength goes down, the bombardment increases, as does the number of carbon 14 atoms.
All living beings assimilate carbon dioxide molecules, a fixed but very small fraction of which contains carbon 14. This assimilation stops upon the death of the organism, thus halting the absorption of any more carbon 14. The atoms of carbon 14 then proceed to decay exponentially, with a half-life of 5,700 years. When much later, an archaeologist examines the remains (fireplace ashes, bones, plant remains), he can date the fossil by comparing the fraction of remaining radiocarbon nuclei to the fraction existing at the time the organism stopped absorbing carbon.
The fundamental hypothesis in these estimations is that the rate of radioactive carbon existing when the organism was living would have been the same as the rate in a similar organism alive today. The ratio of the activities of the fossilized and living bodies then provides an age. The estimation assumes that the rate of formation of atmospheric carbon 14 has not changed since the days when the fossil was alive. This is not entirely true and it is necessary to readjust the time and make corrections.
Dendrochronology and how to improve radioacarbon datation accuracy
For dates that are not too remote, the dating can be improved. Calibration of radiocarbon formation as a function of years is obtained from « dendrochronology », a technique based on trunks of secular. The section of a tree trunk is an extraordinary witness of the plant life. Each ring corresponds to the wood formed in a given year. By counting the rings, one dates the year. By measuring the ring carbon-14 content, one measures the activity of a wood sample of the year in question. Using this information, one tcan check and improve by comparison carbon-14 datings.
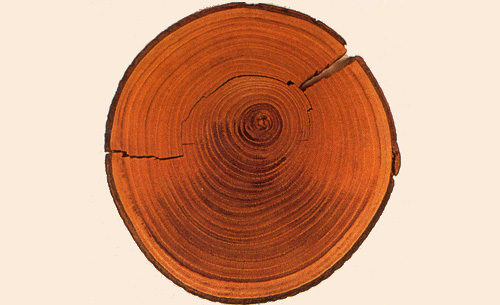
Dendrochronology
Cross-section of a chestnut tree showing its annual rings. Each ring correspond to a year. The measurement of its rings radioacarbon activities gives the evolution of this radioactivity with time. Thanks to this calibration technique, one is able to perform accurate carbon-14 dating up to 11,400 years.
© CNRS
Redwoods and redwoods of California’s forests are used to go back as far as 2000 years ago. Fossil trees provide access to older times. As the thickness of the rings depends on the years climates, characteristic sequences of rings make it possible to compare fossil trees with very old trees which were their contemporaries. Gradually, by cross-checking from one trunk to another, one can connect the sequence of rings up to 11,000 years. By comparing the real age of a ring to its age calculated from its radiocarbon, one obtains the correction to be applied to the ages calculated by carbon-14 dating.
Dendrochronology does not allow us go further than 11,000 years. Beyond, another technique is used to calibrate carbon-14: dating fossil corals using the uranium-thorium method. Calibration is obtained by comparing the ages given by carbon-14 for these corals and the age calculated from the uranium-234 and thorium-230 proportions.
Beyond 20,000 years of age, corrections using uranium-thorium ratio becomes difficult. Geomagnetic calibration methods suggest that between -20000 and -40000 years, the intensity of the Earth’s magnetic field, which serves as a shield against cosmic radiation, was weaker than now. Conversely, carbon-14 production was then significantly higher.
Count carbon-14 atoms and not just their decays.
The measurement of carbon-14 activity requires a sufficiently large fossil sample. Obtaining such a sample can be tricky, since carbon-14 atoms are very rare. There are a trillion times less (10 to the power -12) radiocarbon atoms than ordinary carbon-12 atoms. The radioactivity of a “fresh” gram of carbon is counted in counts per minute. For ancient samples, it may becomes too low for an accurate measure.

ARTEMIS : a facility to count carbon-14 atoms
When the samples to date are very old, the nuclei of carbon-14 become so rare that the observation of their decays becomes impractical. One has to count the carbon-14 atoms themselves. This is done in facilities designed for this purpose, made of a mass spectrograph associated with a small accelerator. Samples of a few milligrams of the vestige to date are introduced in the installation which allows to measure the isotopic ratios of the ordinary carbon and its radioactive isotope. The photograph shows the CEA ARTEMIS facility in Saclay (France).
© ARTEMIS/CEA
One of the key breakthroughs of recent years has been the development of techniques sensitive enough to directly count the carbon 14 atoms present in a sample instead of counting their rare disintegrations. Thanks to a ‘mass spectrometer‘ connected to a particle accelerator, physicists are able to count radiocarbon atoms at the rate of one in 1000 trillion (10 to the power -15), and thus go back 50,000 years in time. The key advantage is to require minute samples of fossil for the dating.
This technique was first implemented in France at the center of the low radioactivity of Gif-sur-Yvette in France with an instrument called Tandetron. It has been replaced since 2004 by Artemis, a mass spectrometer capable of dating each year 4,500 samples of less than a milligram.
Other articles on the subject « Radioactive Dating »
Thermoluminescence
Dating by releasing the energy stored by the radioactivity Many minerals emit light when heated. [...]
Dating in geology
Going back to the distant past In order to date old geological material, geologists rely on radio[...]
Wine Authentication
How to use radioactivity to authenticate a vintage Large quantities of artificial radionuclides f[...]